Regulatory
All the regulatory studies are critically evaluated during study plan preparation. All the necessary regulatory requirements, method selection, and established procedures will be discussed with the sponsor.
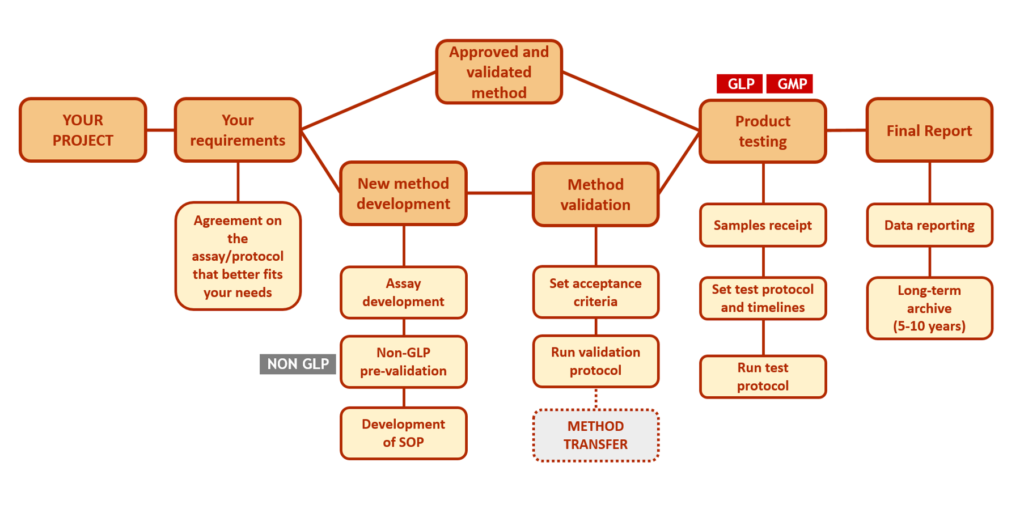
Finalized study plans are executed as agreed with the sponsor.
At Curio Biotech, research studies related to Good Laboratory Practice (GLP) are performed considering the following regulatory guidelines.

• OECD: Organization for Economic Co-operation and Development
OECD Series on Principles of Good Laboratory Practice (GLP) and Compliance Monitoring No. 1
OECD Principles on Good Laboratory Practice
OECD Guidance Document on Good In Vitro Method Practices (GIVIMP)
• OGLP: Ordinance on Good Laboratory Practice of 18 May 2005 (Status as of 1 December 2012), Switzerland
• Environmental & Personnel Protection: Federal Office for the Environment (FOEN)
• EMA (European Medicines Agency) Annex 11: Computerized Systems; FDA (Food and Drug Administration) CFR Title 21, Part 11; AGIT (Swiss Working Group on Information Technology) in a GLP Environment
• ICH: International Conference on Harmonization
Quality
- Ensure that all our deliverables are committed to quality compliances, reproducibility and traceability
- Specified and agreed protocols will be performed by qualified and experienced personnel
- A regular internal audits of all critical processes and continuous improvements in processes and systems to provide consistent quality services to meet sponsor’s requirements
- Qualifying, calibrating, and maintaining equipment that are used to test products
- A stringent supplier selection, qualification and procurement process
- Continuous training of personnel in scientific and quality matters
- Documents archiving 5-10 years (as per legal requirements and request by sponsor)
Project Management